From FMEA to Test & Traceability Matrix
In order to ensure in a traceable and documented manner that each GxP-critical user requirement has been tested as part of qualification (incl. leveraging approach), a traceability matrix is created in a C&Q project for each equipment or its associated URS(s).
With REXS it is very easy to automate this process.
For this purpose, the individual URS requirements are integrated into the REXS FMEA. The risk- mitigating measures defined in the FMEA are supplemented by additional data - for example, a descriptions of the test execution or the acceptance criteria to be met.
This extends the FMEA to a test matrix without much additional effort.
If the test results of the C&Q tests are documented in REXS as well, the FMEA finally expands into a traceability matrix.
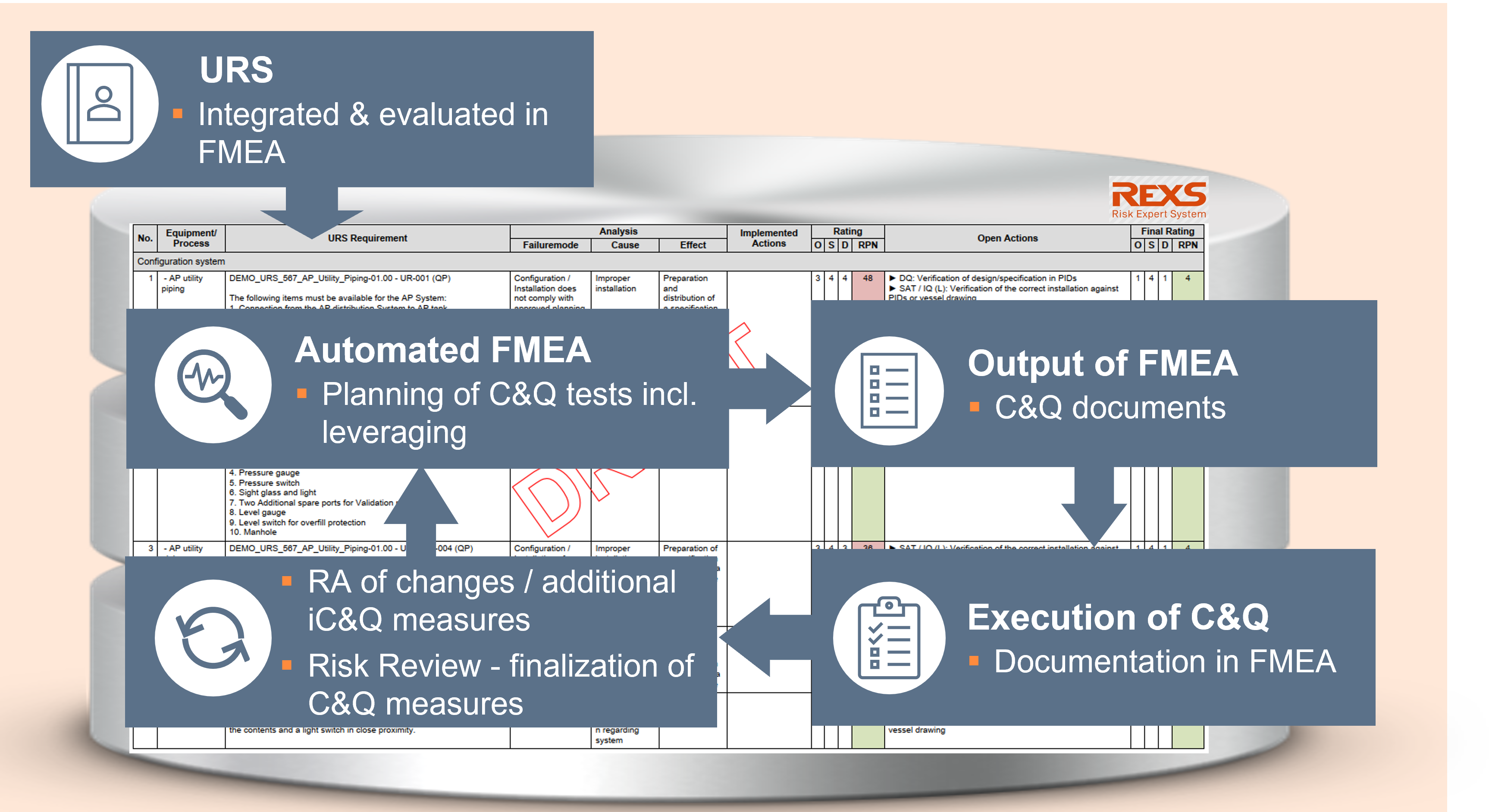
Due to the database's ability to combine and display data from the FMEA and the risk-mitigating measures, the commissioning (FAT/SAT) and qualification protocols (DQ, IQ, OQ, PQ, PPQ, CV, etc.) as well as the test matrix can be easily created "at the push of a button" as an (electronic) document, of course GMP compliant.

![[Translate to English:] [Translate to English:]](/fileadmin/content/REXS/Bilder/header_rexs.png)
![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/a/0/csm_traceabilitytestmatrix_d3600a00a4.jpg)
![[Translate to English:] [Translate to English:]](/fileadmin/content/REXS/Bilder/papierlosevalidierung.jpg)